ChemInform Abstract: Two Step Synthesis of 3-Deoxy-D- or L-glycono-1,4-lactones and 2-O-Alkyl-3-deoxy-D-glycono-1,4-lactones from D- or L-Glyconolactones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
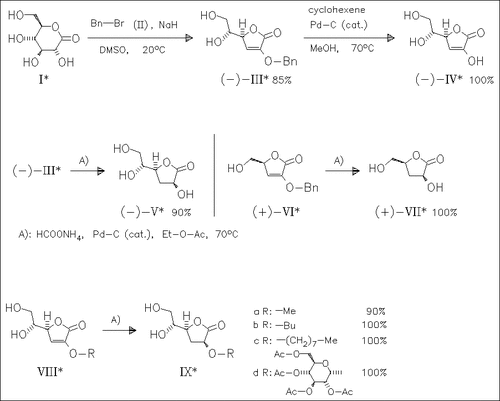
2-O-alkyl-3-deoxy-2-enono-1,4-lactones such as (III), (VI), and (VIII) are efficiently prepared via a regioselective alkylation—elimination procedure and subjected to Pd-catalyzed hydrogen transfer reactions. For 2-O-benzyl derivatives such as (III), the use of cyclohexene as hydrogen donor provides the corresponding debenzylated enonolactones [cf. (IV)], whilst deprotection and stereoselective double bond hydrogenation [→ (V), (VII)] is observed by use of ammonium formate as hydrogen donor. Ammonium formate reduction of 2-O-alkyl substituted unsaturated lactones (VIII) provides the corresponding 2-O-alkyl-3-deoxyglyconolactones [cf. (IX)].