ChemInform Abstract: Versatile Synthesis of myo-Inositol Phospholipid Precursors.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
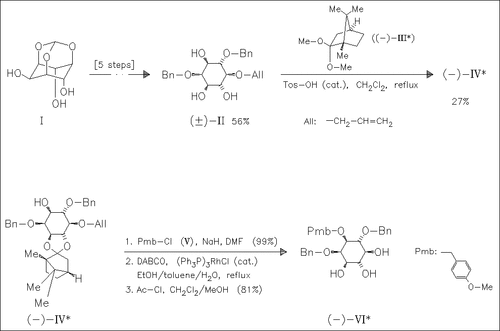
Homochiral myo-inositol derivatives like (VI), possessing either the natural or unnatural ring stereochemistry for inositol phospholipids, are synthesized from racemates (II) using camphor acetals, e.g. (III), in a resolution—protection sequence.