ChemInform Abstract: First Synthesis of Optically Pure Selenuranes and Stereoselective Alkaline Hydrolysis. Their Application to Asymmetric [2,3] Sigmatropic Rearrangement of Allylic Selenoxides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
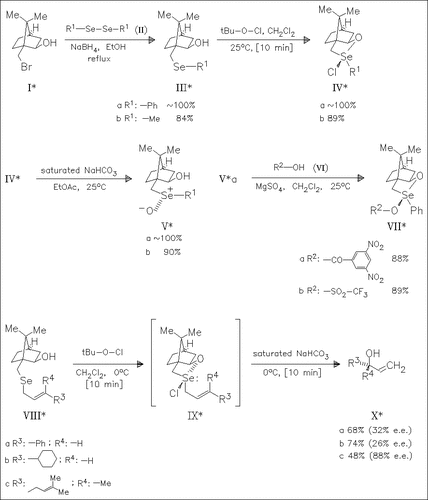
The first chiral selenuranes (IV) are prepared utilizing the 2-exo-hydroxy-10-bornyl group as chiral moiety. Alkaline hydrolysis of (IV) proceeds with complete retention of configuration. Sigmatropic rearrangement of allylic selenoxides (IX) yields allylic alcohols (X) with moderate to good e.e.