ChemInform Abstract: A New Synthesis of Isoselenoureas by Imidoylation of Amines with Selenium and Isocyanides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
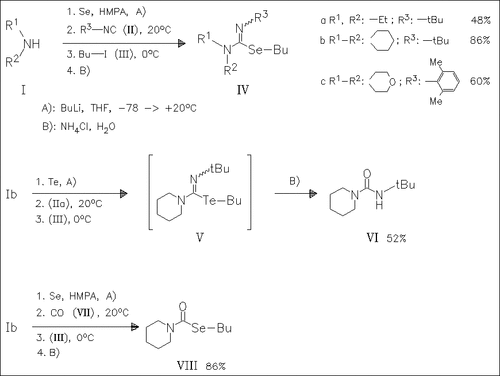
Lithiation of secondary amines followed by transmetalation with selenium and reaction with isocyanides generates lithium selenocarbamidites. They are trapped by butyl iodide to yield isoselenoureas of type (IV). When tellurium is used instead of selenium, the urea (VI) is isolated probably via hydrolysis of the isotellurourea (V) during work-up. Replacing the isocyanide by carbon monoxide results in clean carbonylation to give the selenocarbamate (VIII).