ChemInform Abstract: Chemistry of 5-Oxodihydroisoxazoles. Part 17. Acylation of 5-Oxodihydroisoxazoles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
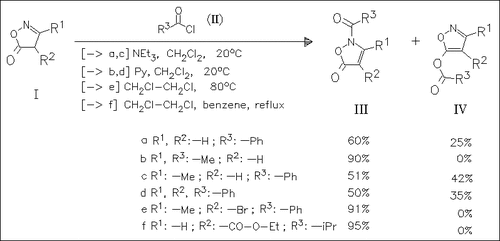
Acylation of isoxazolones (I) yields N- and O-acylated products (III) and (IV), resp., in ratios strongly depending on the nature of the substituents present at the isoxazolone ring. Aliphatic acid chlorides generally give N-acylated products (III), while aroyl chlorides give significant proportions of O-acylated products (IV), if there is no electron withdrawing substituent at C-4.