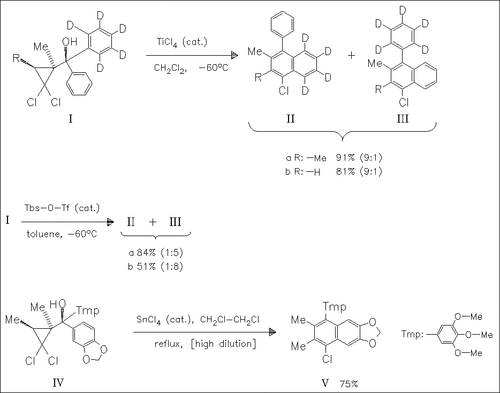
ChemInform Abstract: Regiocontrolled Benzannulation of Diaryl(gem-dichlorocyclopropyl)methanols for the Synthesis of “Unsymmetrically” Substituted α-Arylnaphthalenes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The regioselectivity in the Lewis acid catalyzed benzannulation of the cyclopropane (I) is investigated. Thus, TiCl4 (or SnCl4) favors the deuterated naphthalene (II), whereas Tbs—O—Tf gives the Ph—D5 isomer (III) highly selectively. These effects can be used for the selective synthesis of unsymmetrical naphthalenes such as (V).