ChemInform Abstract: Synthesis of Bicyclo[3.2.1]-octanone Derivatives by Metal Catalyzed Decomposition of α-Diazomethylketones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
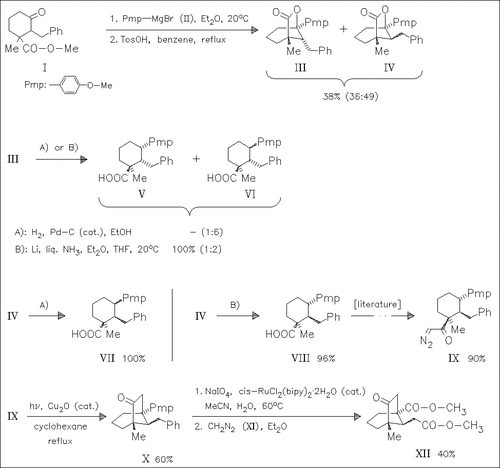
A facile synthesis of bicyclooctanone derivatives [cf. (X), (XII)] involving reductive cleavage of benzylic lactones [cf. (IV)] and carbene insertion of diazoketones by metal catalysis [(IX) → (X)] as the key reactions is accomplished. It is demonstrated, that the stereochemistry of the reductive cleavage of benzylic lactone (IV) depends on the conditions employed. Whilst cleavage using Li/NH3 proceeds with retention of configuration at the benzylic center [→ (VIII)], catalytic hydrogenation provides the inversion product (VII). However, reductive cleavage with Li/NH3 as well as catalytic hydrogenation of C-2-axial benzyl lactone (III) predominantly leads to the product with inverted stereochemistry at the benzylic center (VI). Mechanistical suggestions are discussed.