ChemInform Abstract: Synthesis of Optically Active 2-Siloxycyclopropanecarboxylates by Asymmetric Catalysis. Part 5. Baeyer—Villiger Oxidations of γ-Oxo Esters — Determination of the Absolute Configurations of Methyl 2-Siloxycyclopropanecarboxylates — Interpretation of the Enantioselective Cyclopropanations of Silyl Enol Ethers.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
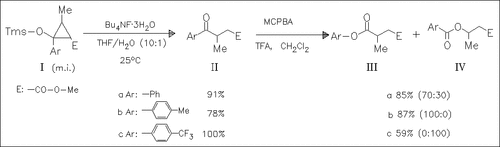
It is demonstrated that racemic model siloxycyclopropanes can be converted into γ-oxobutanoates which on subsequent Baeyer—Villiger oxidation give depending on the electronic properties of the aryl group the products such as (III) and (IV). Application of these reactions on enantiomerically enriched compounds (I) leads to some known chiral products. By comparison with known reference compounds the absolute configuration of the derivatives of type (III) and (IV), and hence those of their cyclopropane precursors can be determined. Further literature data on the optical rotations of related cyclopropanes are collected and compared. On the basis of these results the mechanism of the asymmetric cyclopropanation of silyl enol ethers is discussed.