ChemInform Abstract: The Chemistry of Acylals. Part 1. The Reactivity of Acylals Towards Grignard and Organolithium Reagents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
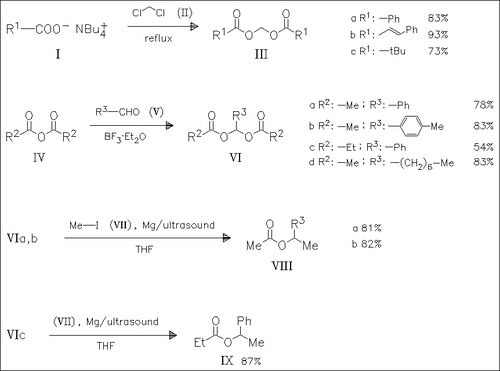
The aldehyde acylals (III) (6 examples) and (VI) (10 examples) are synthesized in order to study their reactivity towards Grignard and alkyl-Li reagents. Generally, Grignard reagents afford better yields of esters than Li reagents. The acylals (III) are inapplicable as substrates for synthetic purposes and lead to complex reaction mixtures with both reagents.