ChemInform Abstract: Ester Derived Titanium Enolate Aldol Reaction: Highly Diastereoselective Synthesis of syn- and anti-Aldols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
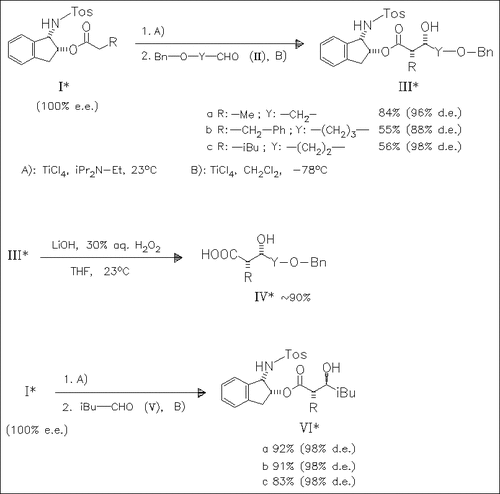
Titanium enolates derived from esters (I) undergo aldol reactions with the bidentate aldehydes (II) yielding the adducts (III) with high syn-diastereoselectivities. In contrast, reactions of the monodentate isovaleraldehyde (V) proceed with excellent anti-selectivity.