ChemInform Abstract: A New Method for the Synthesis of α,α-Difluoro-β-hydroxy Esters Through the Enolization of S-tert-Butyl Difluoroethanethioate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
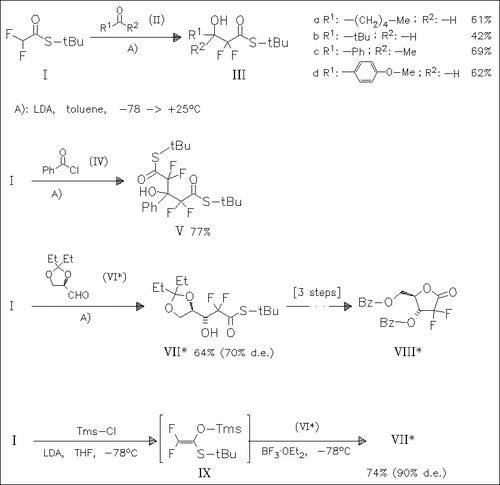
The reaction of the Li-enolate of (I) with carbonyl compounds provides a method for the synthesis of α,α-difluoro-β-hydroxy esters. The Li-enolate adds to the chiral aldehyde (VI) with good diastereoselectivity. The major isomer (VII) can be easily converted to a difluoropentofuranose derivative , a precursor of the novel anticancer agent gemcitabine. Interestingly, the diastereoselectivity of the reaction can be enhanced by use of the intermediate O,S-acetal (IX).