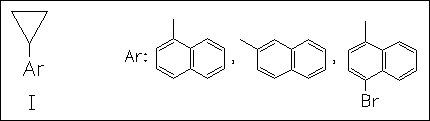
ChemInform Abstract: Radical Ion Probes. Part 6. Origin of the High Intrinsic Barrier to Nucleophile-Induced Ring Opening of Arylcyclopropane Radical Cations.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The radical cations generated from (I) are studied electrochemically. Their anodic oxidation in MeCN/MeOH mainly produces cyclopropane ring opened products. The rate of ring opening is found to be highly sensitive to substituents on the aromatic ring. The results are explained on the basis of a product-like transition state for ring opening wherein the positive charge is located on the cyclopropyl group. The suitability of the reactions as probe for single-electron transfer is discussed.