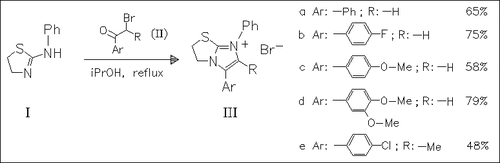
ChemInform Abstract: Reaction of 2-Phenylaminothiazoline with α-Haloketones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The reaction of α-haloketones (II) with aminothiazoline (I) proceeds regioselectively by attack of the halide at the exocyclic nitrogen atom of the aminothiazoline (I) to give the imidazo[2,1-b]thiazolinium bromides (III). The structure of the bromides is supported by spectroscopic and chemical methods.