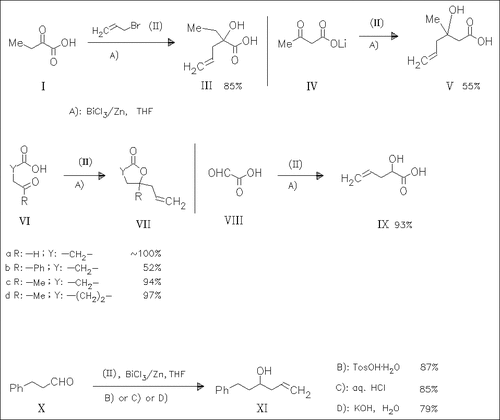
ChemInform Abstract: A Grignard-Type Addition of Allyl Unit to Carbonyl Compounds Containing a Carboxyl Group by Using BiCl3—Zn(0)—Allyl Bromide.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Carbonyl compounds containing a carboxyl group react with allyl bromide to afford the corresponding homoallylic alcohols, e.g. (III), or intramolecular dehydrated lactones such as (VII). The use of BiCl3 is essential in these reactions. The reaction of phenylpropanal (X) with allyl bromide proceeds smoothly under strongly acidic conditions as well as under strongly basic conditions.