ChemInform Abstract: Chiral Oximes in Asymmetric Synthesis. Part 2. Addition of Butyllithium to Benzaldehyde O-(1-Phenylalkyl)oximes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
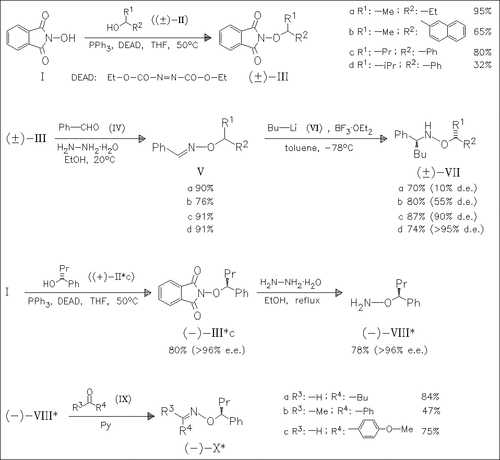
A series of benzaldoxime ethers (V) bearing a chiral auxiliary on oxygen are prepared to investigate the effect of the auxiliary on the diastereoselectivity of the addition of butyllithium to the oxime C=N bond. By increasing the size of the alkyl group R1 in the auxiliary, an increase in the d.e. is observed with best results for the propyl derivatives (Vc) and (Vd). Increasing the size of the aryl group is not advantageous. Enantiomerically pure alcohols such as (R)-(IIc) provide enantiomerically pure oximes (X), which can be used for the synthesis of corresponding optically active hydroxylamine derivatives.