ChemInform Abstract: Studies Towards the Synthesis of Diazonamide A. Unexpected Formation of a 3,4-Bridged Indole.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
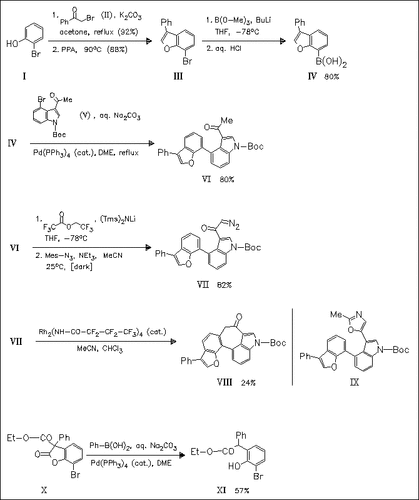
Model studies towards the synthesis of the cytotoxic marine natural product diazonamide A are described. Key step is the Suzuki coupling of an appropriate 3-phenylbenzo(b)furan derivative with 4-bromoindole derivative (V). While the coupling reaction proceeds in excellent yield with the extremely robust benzofuran (IV) as coupling partner, with related benzofuranone (X) the desired coupling product is not obtained. Rh(II) perfluorobutyramide catalyzed cyclization of (VII) does not provide the expected oxazole (IX) but gives rise to the unusual 3,4-bridged indole (VIII).