ChemInform Abstract: Asymmetric Total Syntheses of (+)-Coronafacic Acid and (+)-Coronatine, Phytotoxins Isolated from Pseudomonas syringae Pathovars.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
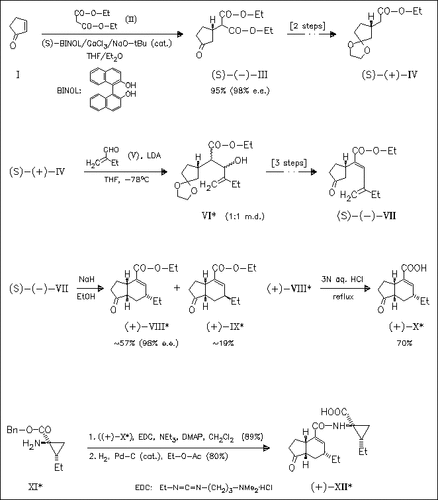
The key steps in the synthesis of the title acid (X) are asymmetric addition of the diester (II) to the enone (I) and diastereoselective cyclization of the ketone (VII). Coupling of (X) with the amino ester ( XI) and subsequent deprotection yields (+)-coronatine (XII).