ChemInform Abstract: Tandem Michael Addition Alkylation of Vinylphosphonates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
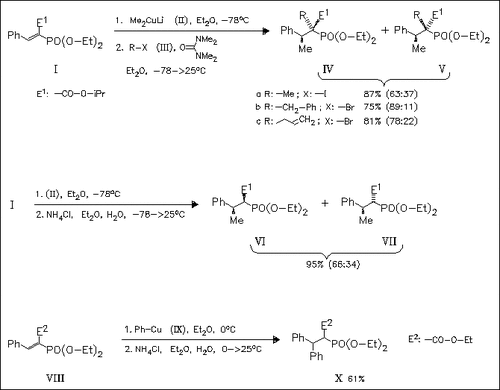
Successive treatment of the vinylphosphonate (I) with Me2CuLi and benzyl or allyl bromide proceeds with good stereoselectivity, whereas with simple alkyl halogenides as well as by quenching of the enolate intermediate with NH4Cl poor selectivity is observed. Alkylation of the enolate formed from the vinylphosphonate (VIII) and PhCu fails, but quenching with NH4Cl gives the diphenyl derivative (X).