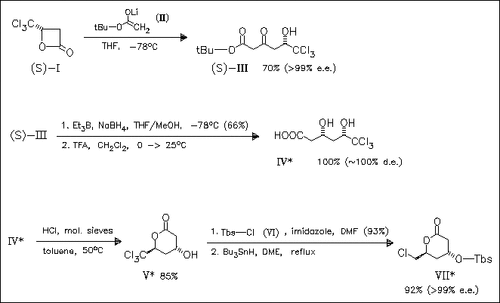
ChemInform Abstract: Facile Synthesis of β-Hydroxy-δ-valerolactone via the Ring Opening Reaction of β-Trichloromethyl-β-propiolactone with Ester Enolates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Key steps in the synthesis of the title compound (V) are the syn- selective reduction of ketone (III) and the subsequent cyclization. Compound (VII) is an excellent precursor for the synthesis of compactin and mevinolin derivatives.