ChemInform Abstract: Nucleophilic α-Addition to Alkynoates. A Synthesis of Dehydroamino Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
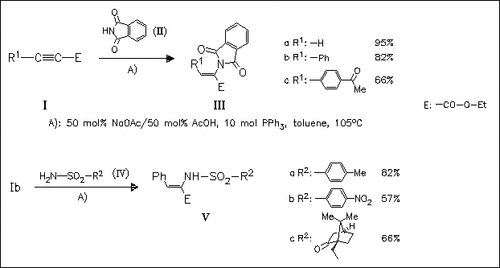
The phosphine induced α-addition of nitrogen nucleophiles to conjugated alkynoates represents a new regioselective mode for the preparation of dehydroamino acids. By the use of alkyl substituted propiolates competitive γ-addition or redox isomerization can also occur. The reaction conditions, mainly the phosphine, infuence the course of reaction.