ChemInform Abstract: Effects of Subjoin Lewis Acid on the Catalytic Asymmetric Allylic Transfer Reactions of Aldehydes Promoted by BINOL-Ti(IV) Complex.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
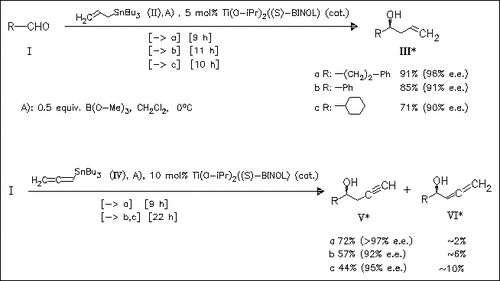
Aldehydes (I) undergo an effective asymmetric addition reaction with tin reagents (II) and (IV) in the presence of catalytic chiral BINOL- Ti(IV) complex and subjoined Lewis acid B(OMe)3. The presence of B(OMe) 3 remarkably increases the rate and catalytic capability; however, the effect is not yet fully understood.