ChemInform Abstract: Substrate-Directed Diastereoselective Hydroformylation. Part 1. Substrate-Directed Diastereoselective Hydroformylation of Methallylic Alcohols - Development of an Efficient Catalyst-Directing Group for Rhodium-Catalyzed Hydroformylation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
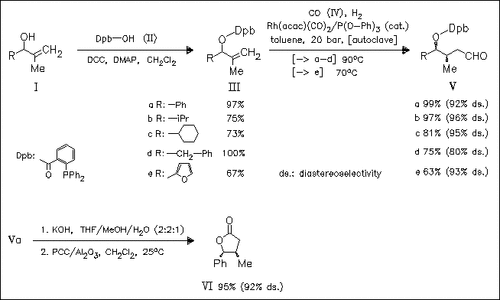
The protection of methallylic alcohols (I) with the o-( diphenylphosphanyl)benzoyl group (Dpb) leads to the development of an optimized, substrate-directed, highly diastereoselective, rhodium- catalyzed hydroformylation method, which provides stereoselective access to acyclic building blocks of type (V) containing two adjacent stereocenters. Subsequent transformation to the corresponding γ- lactones, e.g. (VI), is readily achieved without loss of stereoselectivity.