ChemInform Abstract: Synthetic Studies on Indoles and Related Compounds. Part 41. A General Synthetic Route for 1-Substituted 4-Oxygenated β-Carbolines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
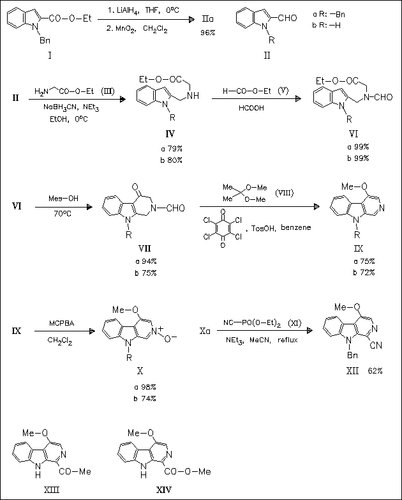
Starting from indole-2-carboxylates the functionalized compounds (VI) are prepared. Their C-3 selective cyclization and functionalization at the C-1 position of the β-carboline nucleus by a modified Reissert-Henze reaction are the key steps in the synthesis to the title compounds (XIII) and (XIV). These compounds can be used as intermediates leading to the general synthesis of this family of compounds.