ChemInform Abstract: Total Synthesis of Dehydrodidemnin B. Use of Uronium and Phosphonium Salt Coupling Reagents in Peptide Synthesis in Solution.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
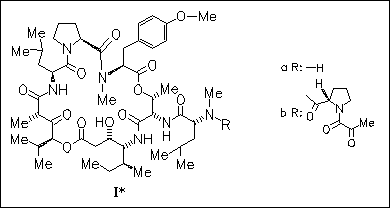
The antiproliferative title compound (Ib) is obtained from didemnin A ( Ia), which was synthesized by 2 routes in 4% and 27% overall yield. The first method is based on the elaboration of a linear heptadepsipeptide incorporating the first amino acid of the didemnin side chain, (R)-N(Me)-Leu. Deprotection of the amino and carboxyl terminii of this linear precursor followed by macrocyclization yields a protected derivative of (Ia). The second route involves the synthesis of the Boc-protected didemnin macrocycle from a linear hexadepsipeptide lacking (R)-N(Me)-Leu. Removal of the Boc group from the macrocycle followed by coupling with Boc-(R)-N(Me)-Leu-OH yields Boc-didemnin A. Extensive use is made of the title coupling agents.