ChemInform Abstract: Simple Methods for the Preparation of Protected Derivatives of D-allo- and L-allo-Threonine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
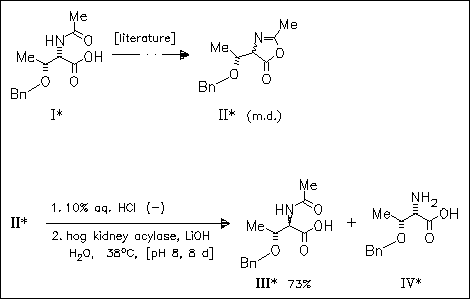
Key step in the synthesis of the title compounds is the enzymatic resolution of threonine diastereomers which are obtained by epimerization of the α-carbon atom in compound (I) via formation of oxazolone derivative (II). This method is developed with a view to the solid-phase synthesis of peptides containing non-proteinogenic amino acids (some yields not given).