ChemInform Abstract: 5(4H)-Oxazolones. Part 10. Acid and Base Effects on the Translactonization Reaction of 4-(2-Oxa-alkylidene)-5(4H)-oxazolones: New Synthesis of 5-Alkylidene-3-benzoylamino-2(5H)-furanones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
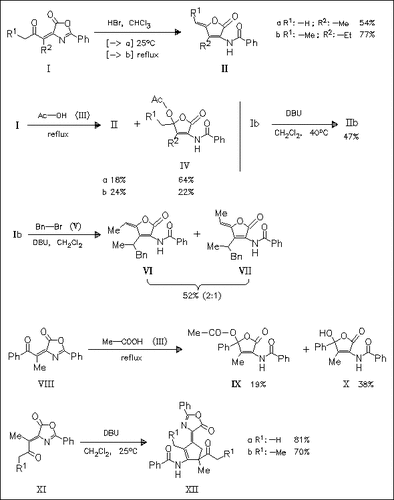
The oxazolones (I) can be easily transformed under acidic conditions into the title furanones (II) with Z-configuration at the exocyclic double bond. The reactions can also be carried out with the Z- oxazolones because they can be transformed into the E-isomers by photochemical isomerization. The same reaction in acetic acid gives besides (II) the furanylacetates (IV). The results in the transformations of the oxazolones in the presence of a base depend on the steric hindrances in the starting compounds.