ChemInform Abstract: Neopentyl Ester Protecting Groups for Arylsulfonic Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
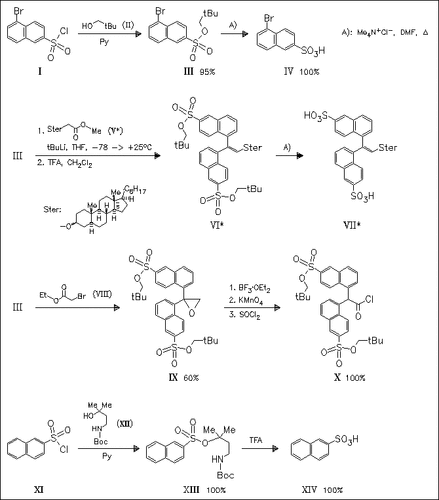
Neopentyl is used as a protecting group for brominated arylsulfonic acids. Its potentials are the high compatibility with a wide range of organic reagents such as vinyl-MgBr, CrO3, NBS/benzoyl peroxide, H2/ Raney-Ni, NaI, aqueous HBr or NaOH, HONH2 and NaH and the easy cleavage in high yields. In addition the Boc-protected N-neopentyl group present in (XII) is developed as protecting group in solid phase synthesis.