ChemInform Abstract: Efficient and Diastereoselective Synthesis of (+)-Goniobutenolide A via Palladium-Catalyzed Ene-Yne Cross Coupling-Lactonization Cascade.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
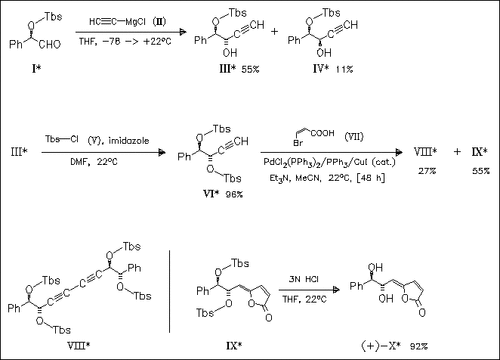
The diastereoselective synthesis of the marginally antitumor (+)- goniobutenolide A (X) is based on the Pd-catalyzed reaction of the terminal acetylene (VI) with (Z)-3-bromopropenoic acid (VII) leading to the lactone with essentially complete control of the exocyclic alkene geometry.