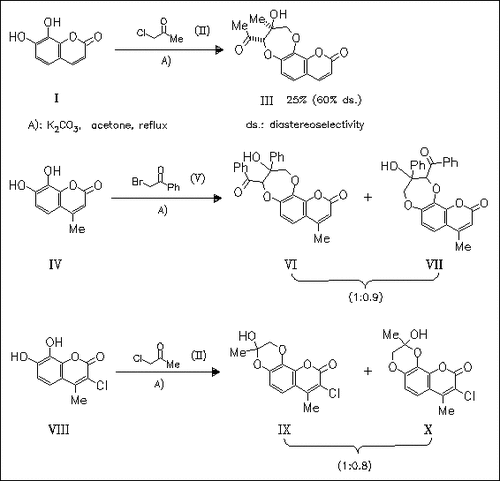
ChemInform Abstract: Reaction of α-Haloketones with ortho-Dihydroxy-2H-1-benzopyran-2- ones. Formation of α-Pyrano-1,5-benzodioxepines - A New Heterocyclic System.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
While the reaction of 7,8-dihydroxybenzopyranone (I) and its 4-methyl derivative (IV) with α-haloketones provides novel α-pyrano- 1,4-benzodioxepines, the 3-chlorinated benzopyranone (VIII) gives . alpha.-pyranobenzodioxane regioisomers under analogous conditions.