ChemInform Abstract: A Practical Method for Epoxidation of Terminal Olefins with 30% Hydrogen Peroxide under Halide-Free Conditions.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
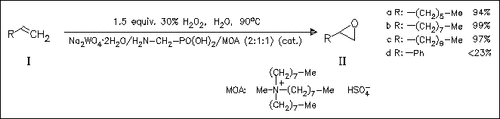
The title method works without the use of organic solvents under phase transfer conditions. Aliphatic olefins (Ia)-(Ic) are epoxidated in high yields, whereas styrene (Id) and its derivatives give only low yields.