ChemInform Abstract: Total Synthesis of (-)-Epothilone A.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
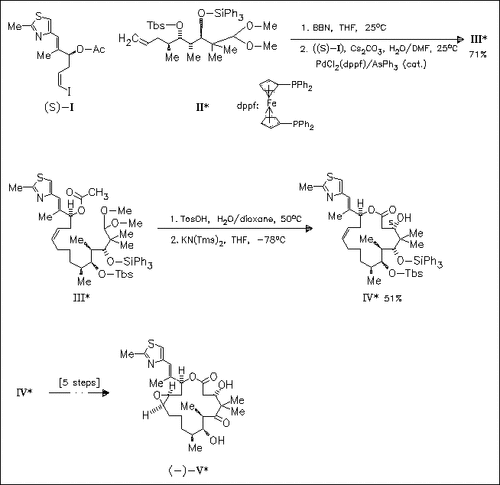
Starting with the chiral building blocks (I) and (II) (preparations are described) coupling product (III) is obtained, the important precursor for the key cyclization. With (III) the aggressive strategy of employing the methyl group of the C-1 bound acetoxy function as the nucleophilic component in the macroaldolization is explored. This highly stereoselective macroaldolization results in the formation of the C-3 (S)-alcohol (IV), from which the title compound (V) is prepared in several steps.