ChemInform Abstract: Total Synthesis of Isodeoxypodophyllotoxin Using the Me3Sn Radical Initiated Carbocyclization.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
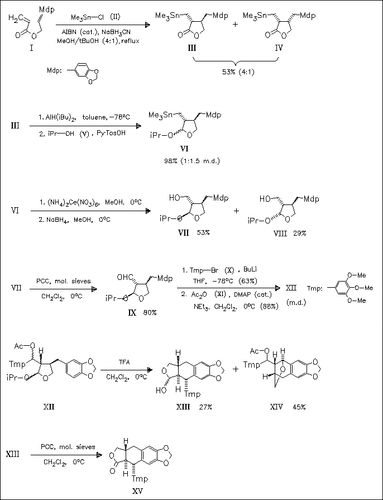
The synthesis of the racemic title compound (XV) uses a trimethylstannyl radical addition to a dienic system for the construction of the lactone portion of the podophylotoxin framework as the key step. Both alcohols (VII) and (VIII) afford the desired product by intramolecular Friedel-Crafts reaction.