ChemInform Abstract: N-Halocarbamate Salts Lead to More Efficient Catalytic Asymmetric Aminohydroxylation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
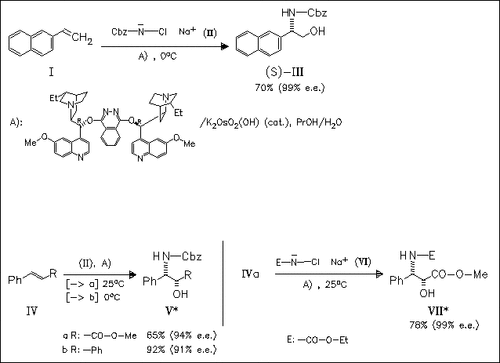
Better enantioselectivity and convenient deprotection make the sodium salt of N-chlorocarbamates (cf. (II)) the oxidant/nitrogen source of choice for the osmium/cinchona alkaloid catalyzed asymmetric aminohydroxylation of olefins. Using the corresponding smaller ethyl carbamate the reaction is superior in terms of rate, enantioselectivity and yield. But despite the greater effectiveness of this reagent, the benzyl analogue is preferred because it can be cleaved easily by hydrogenolysis. This method simplifies the synthesis of a variety of compounds such as unnatural amino acids and other pharmacologically important compounds.