ChemInform Abstract: Excellent Stereocontrol in Intramolecular Buchner Cyclizations and Subsequent Cycloadditions; Stereospecific Construction of Polycyclic Systems.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
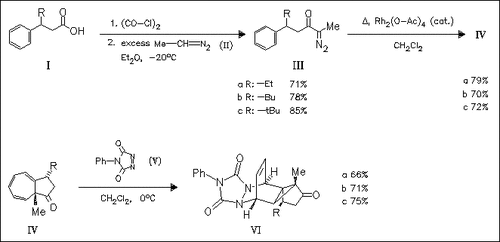
Rh(II)-catalyzed decomposition of the α-diazo ketones (III) results in an efficient intramolecular carbenoid addition to the aromatic ring to furnish the azulenone derivatives (IV) with excellent diastereocontrol. The norcaradiene forms of these azulenones are trapped efficiently with triazolinedione (V) to furnish the pentacyclic systems (VI) as a single diastereomer in each case. The pentacyclic systems (VI) can also be prepared in a highly stereoselective tandem cyclization-cycloaddition process.