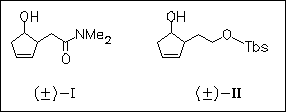ChemInform Abstract: Kinetic Resolution of Racemic 2-Substituted 3-Cyclopenten-1-ols by Lipase-Catalyzed Transesterifications: A Rational Strategy to Improve Enantioselectivity.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The enantioselectivity of the title reaction is not only effected by solvent (diisopropylether is the best) but also by the acylating agent. In case of alcohol (I), enantioselectivity is high with several vinyl esters, especially vinyl butyrate, but low with vinyl trifluoroacetate. In case of alcohol (II) enantioselectivity is excellent in all cases, except with vinyl pivalate. Thus, the acyl group, transiently attached at the active site of lipase catalyst, acts as stereochemical controller.