ChemInform Abstract: The Cyanide-Catalyzed Isomerization of Enol Esters Derived from Cyclic 1,3-Diketones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
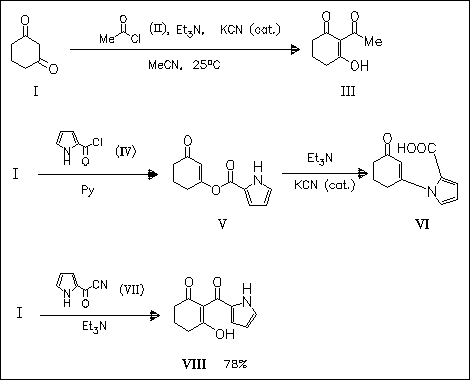
In connection with synthetic studies on the antibiotic pyoluteorin the title reaction is investigated. Thus, acylation of the diketone (I) with aliphatic acyl chlorides, e.g. (II), gives an intermediate O- acylation product which rearranges via the formation of acyl cyanides to the C-acylation product (III). However, application of the sequence to acid chloride (IV) takes another course. The desired C-acylation product (VIII) is directly obtained from (I) and the acyl cyanide (VII) . The aromatization of (VIII) to the corresponding resorcinol, a precursor of pyoluteorin, is not achieved (some yields not given).