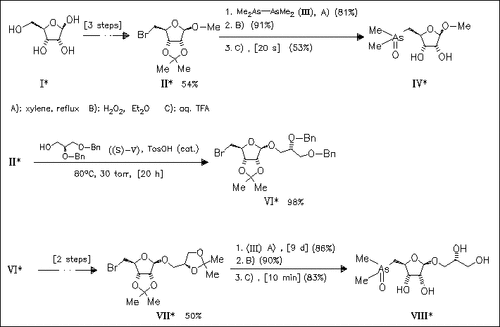
ChemInform Abstract: Synthesis of 1-O-5-Substituted-deoxy-β-D-ribofuranosides with ( CH3)2As and (CH3)2As=O as Substituents at the 5-Position and a Methyl or 2′,3′-Dihydroxypropyl Group as the Aglycone in the 1-Position.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The multi-step reaction sequence described provides a viable route for the preparation of multigram quantities of arsenic-containing ribofuranosides such as (IV) and (VIII) starting with the inexpensive D-ribose (I) and employing tetramethyldiarsine (III). Dimethyl(ribosyl) arsine oxides are the major arsenic compounds from marine algae species.