ChemInform Abstract: An Extremely Mild and General Method for the Construction of 1,2-trans- β-Glycosidic Linkages via Glycopyranosyl Diethyl Phosphites with Participating Groups at C-2.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
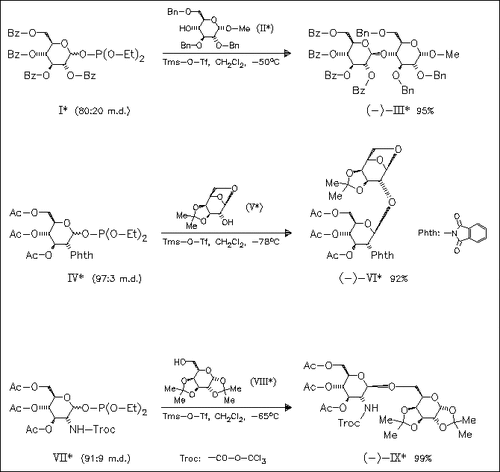
Tms-O-Tf-mediated glycosidation of glycosyl phosphites bearing participating groups such as benzoate (cf. (I)), phthalimido (cf. (IV)) or trichloroethyl carbamate (cf. (VII)) at C-2 constitutes an extremely mild and general method for the stereocontrolled construction of 1,2-trans-β-glycosidic linkages.