ChemInform Abstract: Hydride Addition to 1,2-Anhydrosugars: A Highly Stereoselective Route to Anhydroalditols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
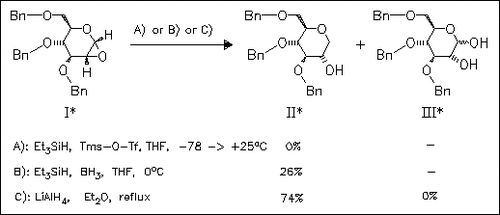
The stereoselective hydride addition reaction to the 1,2-anhydrosugar ( I) is investigated under different reaction conditions. Surprisingly Lewis acid catalyzed addition of triethylsilane gives only the unwanted diol (III) (conditions A) or low yield of (II) (conditions B). However, direct reduction with lithium aluminium hydride yields the desired (II) exclusively.