ChemInform Abstract: Azole Endothelin Antagonists. Part 1. A Receptor Model Explains an Unusual Structure-Activity Profile.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
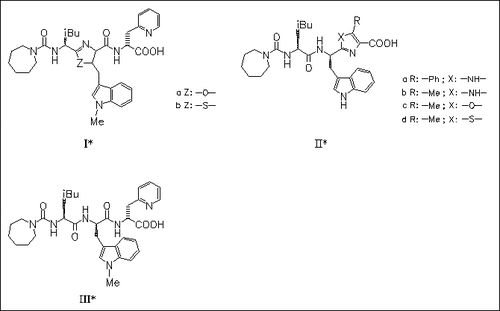
Central (4 examples) and terminal (21 examples) dipeptide surrogates, e.g. (I) and (II), resp., of the pseudotetrapeptide FR-139317 (III), a potent and highly selective antagonist of the endothelin-A (ETA) receptor of poor oral absorption characteristics, are prepared. Some of the resultant analogues are also ETA-selective antagonists, but show a structure-activity profile substantially different from that of the peptidic series, particularly with regard to the requirements of the side chain group that has been incorporated into the heterocycle. The nature of the heterocycle has profound effects on the activity of the compounds. The results are rationalized through examination of a 3D model of ET ligand-receptor binding.