ChemInform Abstract: Synthesis of Carbocycles via Intramolecular Conjugate Additions: Total Syntheses of Axane Sesquiterpenoids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
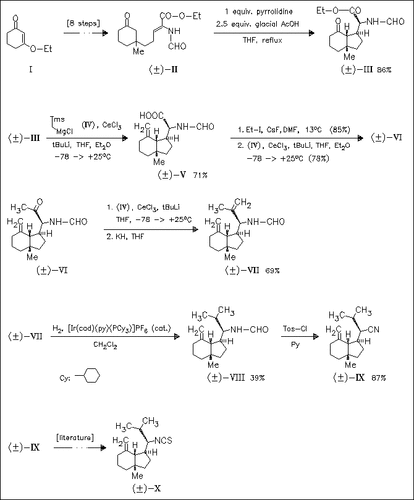
The key steps in the synthesis of the sesquiterpenoids (VIII)-(X) are highly diastereoselective intramolecular cyclization of the unsaturated ester (II) yielding the perhydroindan (III) and a series of cerium-mediated olefinations.