ChemInform Abstract: Stereocontrolled Synthesis of E-Homoallylic Sulfides with 1,4,5 Related Chiral Centres Using the (2,3) Sigmatropic Rearrangement of Sulfonium Ylides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
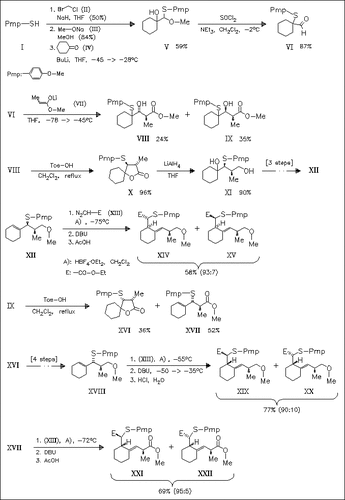
E-Homoallylic sulfides with 1,4,5 related chiral centers such as (XIV), (XIX), and (XXI) are synthesized in a stereocontrolled way by (2,3) sigmatropic rearrangement of the ylides generated in situ from the allylic precursors (XII), (XVII), and (XVIII). The stereochemistry of these precursors is introduced by aldol condensation of the aldehyde ( VI) with the enolate (VII). Lactonization (→ (X), (XVI)) with 1, 2-arylsulfanyl migration converts the aldols stereospecifically into the allylic precursors.