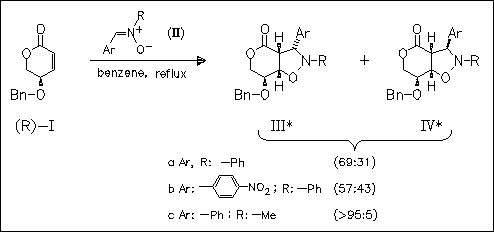
ChemInform Abstract: Stereoselectivity of Nitrone Addition to (R)-4-O-Benzyl-4-hydroxy-2- penten-5-olide.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The reaction of the optically active title lactone (I) with achiral as well as chiral nitrones results in the regioselective formation of two diastereomeric isoxazolidines. In all cases studied, N-methylnitrones react with higher diastereoselectivity than the corresponding N-phenyl analogues.