ChemInform Abstract: Why Do Catalytic Quantities of Lewis Acid Generally Yield More Product than 1.1 Equiv. in the Intramolecular Diels-Alder Reaction with a Furan Diene? Part 2. AM1 Calculations and Mathematical Simulation of the Equilibria.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
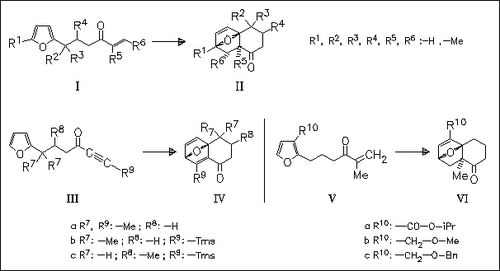
Heats of formation and reaction are obtained using AM1 calculations for 26 intramolecular Diels-Alder reactions of furans of the types (I), (III), and (V). The results support the hypothesis that catalytic quantities of Me×AlCl2 give higher yields than 1.1 equiv. Lewis acid.