ChemInform Abstract: Stereo- and Enantiocontrolled Synthesis of (+)-Juvabione and (+)- Epijuvabione from (+)-Norcamphor.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
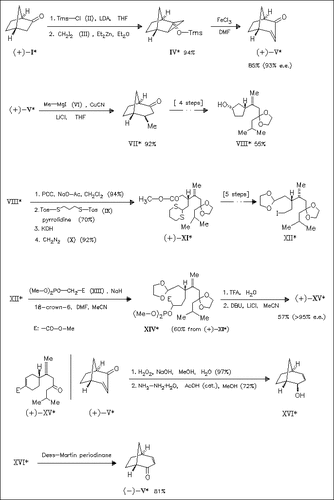
The synthesis of the interesting optically active bicyclic ketone (+)-( V) and its enantiomer (-)-(V) is described starting from (+)- norcamphor (+)-(I), derived from the commercially available (+)-endo norborneol. The usefulness of these synthons are shown by the stereocontrolled synthesis of (+)-juvabione (+)-(XV) in which the key step is the convex face selective 1,4-addition of a Grignard reagent leading to ketone (VII). is the key step. Via carbonyl transformation the enantiomer (-)-(V) is obtained, which in an identical approach as for (+)-(XV) gives the other title compound.