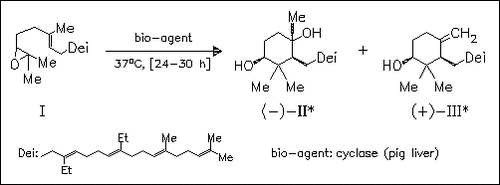
ChemInform Abstract: Substitution of the Methyl Groups with Ethyl Groups at C-10 and C-15 of 2,3-Oxidosqualene Halts the Enzymatic Reaction of Oxidosqualene- Lanosterol Cyclase at the Monocyclic Ring Stage.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The cyclization of 40 mg of the oxidosqualene (I) stops at the stage of monocyclic compounds giving 4 mg of (-)-(II) and 5 mg of (+)-(III) after 45% conversion based on one enantiomer of the racemic (I). No conversion is observed with cyclase derived from baker′s yeast. The influence of the title substituents is discussed.