ChemInform Abstract: Total Synthesis of Granditropone, Grandirubrine, Imerubrine, and Isoimerubrine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
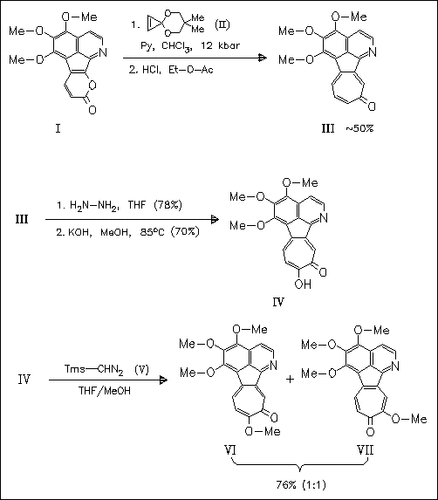
(4 + 2) Cycloaddition reaction of the α-pyron (I) with the cyclopropenone ketal (II) followed by acid treatment predominantly provides granditropone (III) the key intermediate tropone for the naturally occurring title tropoloisoquinolines. Regioselective hydroxylation of (III) gives grandirubrine (IV) and O-methylation yields both imerubrine (VI) and isoimerubrine (VII), which can be separated by SiO2 chromatography.