ChemInform Abstract: Asymmetric Synthesis of 6-Deoxy-D-allonojirimycin, -D-fuconojirimycin and Their 1-Deoxy Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
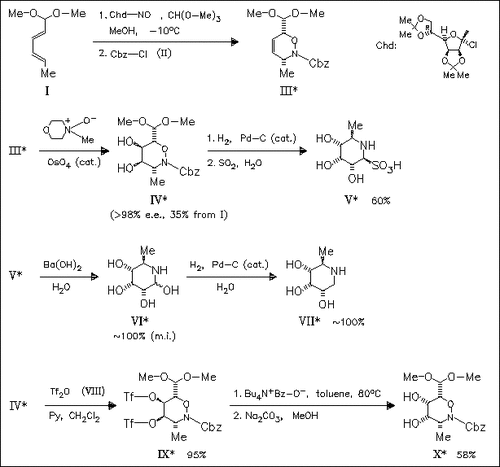
The key step in the asymmetric synthesis of piperidine aminosugars, i. e. the D-allonojirimycin derivatives (V), (VI), and (VII), is the hetero-Diels-Alder reaction of the chiral derivative Chd-NO of D- mannose with the diene (I) followed by catalytic osmylation. Double configurational inversion of triflate (IX) gives access to the non- natural D-fuconojirimycin series (X).