ChemInform Abstract: A Mild Preparation of Amino Acid N-Acylsulfonamides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
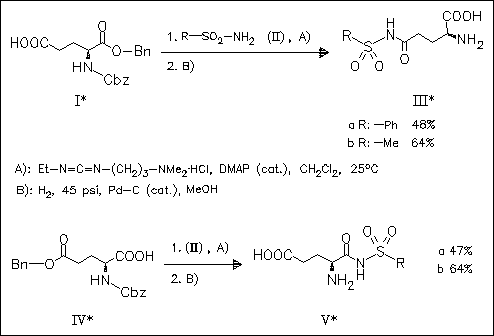
Condensation of N-protected amino diacid monobenzyl esters (I) and (IV) with the sulfonamides (II) followed by catalytic hydrogenolytic ester cleavage provides a mild preparation of the amino acid N- acylsulfonamides (III) and (V). The sequence is an improvement over existing methods. In particular, the γ-substituted glutamic acid acylsulfonamides (III) are obtained without competitive formation of pyroglutamates.