ChemInform Abstract: Saponaceolides: Differential Cytotoxicity and Enantioselective Synthesis of the Right-Hand Lactone Moiety.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
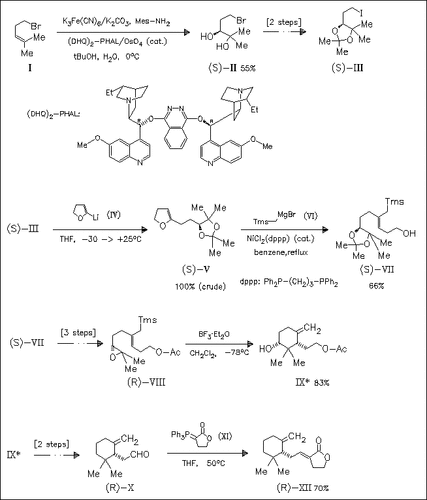
The synthesis of right-hand moiety (XII) of the cytotoxic triterpene saponaceolide B is conducted via the iodide (III), obtained in 3 steps from commercially available (I) or in 8 steps from (S)-malic acid. The mean graph profiles of (XII) do not match the characteristic patterns of differential cytotoxicity of saponaceolides A and B in the NCI human disease-oriented tumor screening panel.